User:Emily Joyce/Sandbox 1
From Proteopedia
Contents |
Introduction
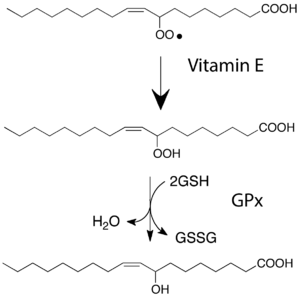
Glutathione peroxidases are a family of enzymes that have antioxidant properties.[1] It fights against oxidative stress by removing reactive oxygen species (ROS) from the cell.[1] Glutathione peroxidase (GPx) can convert hydrogen peroxide to water using glutathione (2GSH + H2O2 → GS–SG + 2H2O), and can reduce peroxide radicals to their corresponding alcohol forms.[2] To do this, GPx utilizes glutathione, glutathione reductase, and cofactors FAD and NADPH.[3] Interestingly, GPx in plants typically rely on thioredoxin instead of glutathione as its electron donor to reduce ROS.[1] [4] Being able to utilize both thioredoxin and glutathione as substrates is not very common, generally speaking, but it has been seen before in both thioredoxin-dependent and glutathione-dependent antioxidant systems (for example, Thioredoxin Reductase from Karenia brevis).[1],[5] Additionally, plants often have cysteine in their active site instead of selenocysteine found in most other GPx homologues.[1] [6] Having cysteine in the active site typically reduces the catalytic efficiency of the enzyme in comparison with its selenocysteine-containing counterparts. Even though plant GPxs are slower, they can reduce wider variety of ROS, as they have lower substrate specificity.[1]
This structure, 2P5R, is the oxidized form of glutathione peroxidase 5 from Populus trichocarpa x Populus deltoides (PtGPX5), from a paper entitled “Crystal Structures of a Poplar Thioredoxin Peroxidase that Exhibits the Structure of Glutathione Peroxidases: Insights into Redox-driven Conformational Changes”.[1][1] At the time of this publication, there were only six crystal structures of GPxs, all of which were mammalian.[1][7][8][9]This paper was ground-breaking, as it provided the first GPx structures not from mammals. Black cottonwood poplar was chosen as the model organism because at the time, its full genome had recently been released and it had six GPX genes.[1] PtGPX5 got classified as a GPx-5, the category of GPxs which are not selenoproteins.[1]
|
Structural highlights
Oxidized PtGPX5 was crystallized as a dimer in the asymmetric unit, with a resolution of 2.45Å.[1] The oxidized conformation of PtGPX5 has less defined regions than the reduced form, especially between residues , where there was barely any electron density in one of the chains.[1] As with any protein model, there is some level of interpretation. These researchers took the electron density and sequence of the flexible loop () from the chain they could see and applied it to the loop containing residues 77-84 which they couldn’t see, keeping allowable Ramachandran conformations in mind.[1] This inconveniently contains Cys92, a residue involved in catalysis/disulfide bond formation.[1] They also predicted the presence of five calcium ions in the oxidized form, since it was crystallized with calcium chloride.[1]
PtGPX5 is structurally similar to animal GPxs, as it is a dimer and has a β-sheet core.[1] Each chain has 170 amino acids and 19.36kDa.[1] Structurally, PtGPX5 is quite similar to other GPxs, varying by about 1Å.[1] PtGPX5 is missing an N-terminal oligomerization region and a region that corresponds to the mammalian GPxs that are tetramers.[1] One notable difference between PtGPX5 and mammalian GPxs is that PtGPX5 has an overall negative charge on the protein’s surface, while mammalian GPxs are more neutral.[1]
Each subunit has the classic thioredoxin fold, which is a .[1] Interestingly, this is not a true ten-stranded β-sheet as seen in mammalian GPxs, as there is a 6Å distance between two of the strands, preventing hydrogen bonding.[1] When the oxidized and reduced conformations are superimposed, they deviate by 4.02Å, primarily in the α1-and α2-helices.[1] In the α1-helix of the oxidized form, there is a little unwinding around catalytic , which results in a small local arrangement.[1] Then, α2-helix completely unwinds to form a long flexible loop containing another catalytic cysteine ().[1] If α1-and α2-helices are excluded, then the oxidized and reduced conformations line up very well, varying only by 0.525Å.[1]
The same dimerization pattern was observed in both the oxidized and reduced forms (which required different crystallization conditions).[1] The s of both forms were about the same size and contained a 3:2 ratio of non-polar to polar atoms.[1] This dimerization interface includes the C-terminus, which is different than previously crystallized GPx structures.[1] The dimerization interface is stabilized by hydrogen bonds involving the polar side chains and van der Waals interactions between the hydrophobic and aromatic side chains.[1]
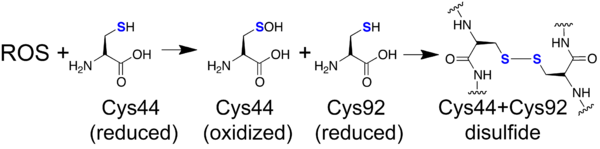
Mechanistically, it is proposed that Cys44 scavenges the ROS and in turn gets oxidized to sulfenic acid.[1] Then, Cys92 quickly reacts with Cys44-SOH to create a disulfide bond.[1] In the oxidized form of PtGPX5, Cys44 of the α1-helix and Cys92 of the flexible loop (α2-helix when reduced) form a disulfide bond.[1] The causes the regions the involved cysteines are in to turn towards each other, shortening the distance between the two cysteines by 12.1Å in comparison to the reduced form.[1] It is proposed that both of these cysteines can also form disulfide bonds with poplar thioredoxin’s Cys38.[1] The electron density of the active site is much better in the reduced form, but the disulfide bond is very evident in the oxidized form. Because of this, it is difficult to speculate the exact local environment around the active site, although the authors propose that nearby tryptophan residues may play a role in substrate recognition.[1] Additionally, it is known from the sequence and from the reduced form that Cys92 is in a very negatively-charged region.[1] This is interesting because this is not that case in mammalian GPxs, but further theories about its relevance cannot be deduced due to the lack of electron density in the flexible loop of the oxidized structure.[1]
Crystallization Methods
PtGPX5 was crystallized using selenomethionine methods to help with phasing issues.[1] Crystals of oxidized PtGPX5 were grown over two days in 0.1M Tris-HCl (pH 8.5), 25% (w/v) PEG 4000, and 0.2M calcium chloride, whereas crystals of the reduced form took a week to grow in 0.1M HEPES (pH 7.5), 0.05M cadmium sulphate hydrate, and 1.0M sodium acetate.[1] Oxidized PtGPX5 was trigonal and designated form II, meaning that it is part of the P3121 space group and has cell dimensions of a=71.6Å and c=48.1Å.[1] There are two subunits per asymmetric unit.[1]
Summary
Glutathione peroxidases are part of the antioxidant network that minimizes the buildup of ROS in a cell. PtGPX5 was the seventh GPx to be crystallized and to have the structure determined.[1] Although GPxs don’t typically use thioredoxin as a substrate, this one does.[1] It is structurally similar to animal GPxs and has a thioredoxin fold. Its dimerization interface remains conserved regardless of oxidation state and of crystallization methods.[1] It was easier to crystallize PtGPX5 in the oxidized state compared to the reduced state.[1] The greatest conformational changes occur when it is switching redox states, as the α2-helix completely unfolds.[1] It becomes a flexible loop when Cys44 gets oxidized and Cy92 subsequently forms a disulfide with it.[1]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 1.23 1.24 1.25 1.26 1.27 1.28 1.29 1.30 1.31 1.32 1.33 1.34 1.35 1.36 1.37 1.38 1.39 1.40 1.41 1.42 1.43 1.44 1.45 1.46 1.47 Koh CS, Didierjean C, Navrot N, Panjikar S, Mulliert G, Rouhier N, Jacquot JP, Aubry A, Shawkataly O, Corbier C. Crystal structures of a poplar thioredoxin peroxidase that exhibits the structure of glutathione peroxidases: insights into redox-driven conformational changes. J Mol Biol. 2007 Jul 13;370(3):512-29. Epub 2007 Apr 19. PMID:17531267 doi:http://dx.doi.org/10.1016/j.jmb.2007.04.031
- ↑ Fanucchi, M.V. Chapter 11 – Development of Antioxidant and Xenobiotic Metabolizing Enzyme Systems. The Lung: Development, Aging, and the Environment, Second Edition; Harding, R., Pinkerton, K.E.; Academic Press, 2014; 223-231.
- ↑ Higuchi, M. Wheat and Rice in Disease Prevention and Health-Benefits, risks and mechanisms of whole grains in health promotion. Elsevier; Watson, R., Preedy, V.R., Zibadi, S.; Academic Press, 2014; 547-557.
- ↑ Burk, R.F.; Hill, K.E. Biotransformation. Comprehensive Toxicology, 2010.
- ↑ Colon, R.; Wheater, M.; Joyce, E.J.; Ste.Marie, E.J.; Hondal, R.J.; Rein, K.S. The Marine Neurotoxin Brevetoxin (PbTx-2) Inhibits Karenia brevis and Mammalian Thioredoxin Reductases by Targeting Different Residues. J. Nat. Prod. 2021, 84 (11), 2961-2970. DOI: 10.1021/acs.jnatprod.1c00795
- ↑ Ursini, F.; Maiorino, M. Glutathione Peroxidases. Encyclopedia of Biological Chemistry, Second Edition; 2013.
- ↑ PMID: PMID: 6852035
- ↑ Ren B, Huang W, Akesson B, Ladenstein R. The crystal structure of seleno-glutathione peroxidase from human plasma at 2.9 A resolution. J Mol Biol. 1997 May 23;268(5):869-85. doi: 10.1006/jmbi.1997.1005. PMID:9180378 doi:http://dx.doi.org/10.1006/jmbi.1997.1005
- ↑ Grignard E, Morin J, Vernet P, Drevet JR. GPX5 orthologs of the mouse epididymis-restricted and sperm-bound selenium-independent glutathione peroxidase are not expressed with the same quantitative and spatial characteristics in large domestic animals. Theriogenology. 2005 Sep 1;64(4):1016-33. doi:, 10.1016/j.theriogenology.2005.01.008. Epub 2005 Mar 5. PMID:16054503 doi:http://dx.doi.org/10.1016/j.theriogenology.2005.01.008