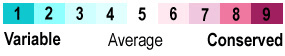Salmonella
FlhB from Salmonella typhimurium consists of 383 amino acids. The cytoplasmic domain 219-383 (length 165, 43% of full length) was crystallized. The resulting model 3b0z includes coordinates for residues 229-353 (length 125, 76% of the crystallized length). The asymmetric unit contains a single molecule ().
Alpha Helices,
Beta Strands
As explained in the publication, the position of the long alpha helix appears to be stabilized by
crystal contacts (not shown).
The chain is . This is believed to be autocatalytic cleavage involved in the transition of the export apparatus from hook to filament mode. Mutations that prevent this cleavage render the bacteria non-motile.
Aquifex
FlhB from the thermophile[1] Aquifex aeolicus is shorter, 350 residues (vs. 383 for S. typhimurium), with 32% sequence identity. Residues 213-350 (length 138) were crystallized, and the resulting model 3b1s has in the asymmetric unit. The molecule displayed in the comparison in the next section, with chains designated C and D, was chosen because it has the lowest average temperature factor (66.2, vs. 84.7 and 72.6 for A,B and E,F respectively). It has coordinates for 232-337 (length 106, 77% of the crystallized segment), cleaved at NPTH between Asn263 and Pro264.
Comparison
The FlhBc Salmonella 3D structure is very similar to that of Aquifex. 102 alpha carbons align with an RMSD of 1.0 Å. Their FlhB's have 32% sequence identity.
, then click the button below to do a structural alignment.
Salmonella vs. Aquifex.
Charge
The (S. typhimurium) show a cluster of positive charges on one side, referred to in the publication as a "positively charged cleft".
Anionic (-) / Cationic (+) / Histidine
However, only two of the positively charged residues in this cleft region are conserved: Arg320 (ConSurf level 9, maximum), and Arg245 (Consurf level 8). This is shown in another scene below under Evolutionary Conservation.
Loop 281-285
Both FlhBc structures have a . Despite the fact that this loop is not conserved (see below), its deletion abolished motility. Mutation of the loop to PPPPP reduced motility, while mutation to AAAAA had no effect. The reductions in motility correlated with reductions in secretion of hook protein FlgE and filament protein FliC.
Flexibility of N-Terminus
Molecular dynamics simulations suggested that the mutations in 281-285 reduced flexibility of the N-terminal alpha helix, and hence that such flexibility may be important for function. Indeed, 226-267 (the N-terminal helix is 229 to about 262) is predicted to be intrinsically disordered[2]. The observed formation of a helix seems likely to depend upon stabilization by crystal contacts mentioned above.
Evolutionary Conservation
was calculated by the ConSurf Server.
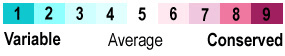
There is a prominent strip of conserved residues on one side of the long N-terminal alpha helix. As mentioned above, the auto-catalytically cleaved segment, including 269 and 270, is highly conserved. In contrast (as mentioned above) the protruding loop 281-285 (which is necessary for function and suggested to be important for flexibility of the N terminus) is not conserved.
Conservation of A. aeolicus FlhBc (not shown) was similar, especially in the regions mentioned above.
Turning to the positive charges discussed above, All atoms are colored by conservation. With spinning off, touch any sidechain to report its identity.

![]()