Introduction
Reverse transcriptase (RT) or RNA-dependent DNA polymerase transcribes single-stranded RNA into double-stranded DNA. HIV-1 RT is from the human immunodeficiency virus and is a heterodimer of P66 and P51 subchains. P15 is its RNAse H domain. There are two types of inhibitors for RT: NNRTIs are the non-nucleoside inhibitors, and NRTIs are the nucleoside inhibitors. Being the protein that gives their name to Retroviruses, Reverse Transcriptase is, along with Protease and Integrase, the most important part of the protein system involved in the process of infection and reproduction for viruses like HIV, MuLV and AMV. RT has the unusual property of transcribing ssRNA into dsDNA going against the Central Dogma of Molecular Biology.
Since its discovery in 1970, the study of its properties and mechanisms of action have been of high interest among the scientific community due to the unique properties that makes it an important medical target enzyme and important tool for genetic engineering applications like RT-PCR in the construction of cDNA libraries. See also
Reverse Transcriptase is one of the CBI Molecules being studied in the University of Massachusetts Amherst Chemistry-Biology Interface Program at UMass Amherst (see HIV Reverse Transcriptase (UMass Chem 423 Student Projects 2011-2)) and on display at the Molecular Playground.
Structure
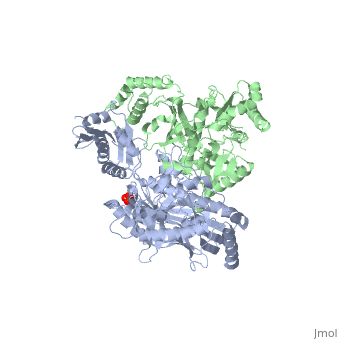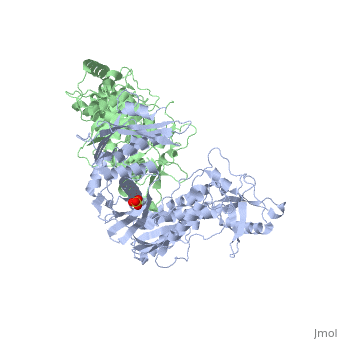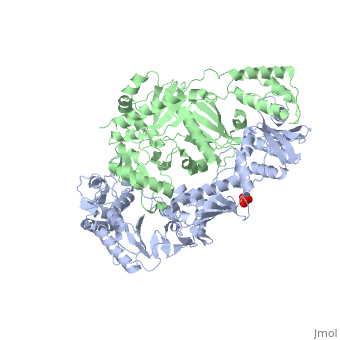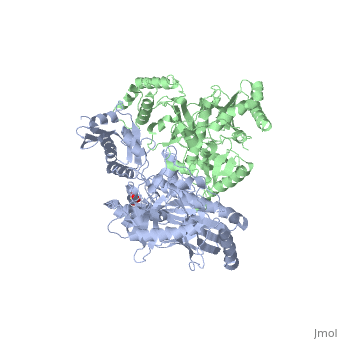
This hand-like protein has an usual length of 1000 residues (560 in Chain A (shown in red) and 440 for B (shown in green)), a third of them involved in alpha helices and almost a quarter involved in beta sheets, showing α+β domains. has an usual weight of 66KDa whereas is around 51KDa. These monomers are derived from the same gene, but p51 lacks the amino acids of one active site and has a different tertiary structure conformation compared to p66. Because of this, p51 is enzymatically inactive[1].
There are five distinct structures within the p66 subchain that are used to describe the functions of RT: the fingers (residues 1–85 and 118–155), the palm (residues 86–117 and 156–236), the thumb (residues 237–318), the connection (319–426), and the RNase H (residues 427-end). The palm contains the main active site (residues 110, 185-186)[2].
Function
As a RNA-dependent DNA Polymerase, Reverse Transcriptase is able to recognize the initial RNA, transcribe it to ssDNA, cleave the remaining RNA and then build up the dsDNA. To do this the protein has two active catalytic zones. Chain A has the that consist of two finger-like domains: one of them recognizes the initial nucleic acid by h-bond interactions with phosphate groups of the side chains, then both domains make a conformational change closing the recognition hole to allow the second domain with the support a coordination system to begin the transcription process adding the specific DNA nucleotides. This change is allowed by a between the two previous domains; it is used as a common pharmaceutical target site in order to prevent the change and therefore inhibit activity. This zone is the only zone of Chain A that has non-conserved aminoacids, giving the virus more drug resistance[3]
Link to Consurf Data Base for PDB Entry: 1JLB.
As the same rate that the polymerization process occurs, the other active site known as the cleaves RNA, releasing the ssDNA that comes again through the Polymerase active site to become dsDNA (all this with a coordinative system, that allows non-specific recognition, just with phosphates). Finally, Chain B, despite the similar aminoacid sequence with Chain A, has no enzymatic activity; its function is possibly to stabilize and interact with both active sites by varying the length between them in order to synchronize both functions.
This is the most general idea of the mechanism of action of Reverse Transcriptase; however the process remains unclear and new approaches are being reported [4].
One of the principal issues about this protein compared to usual DNA polymerase (besides to the similarity with the Klenow fragment), is the lack of a correction mechanism (usually made by DNA PolIII in the DNA Polymerase); this deficiency increases the number of errors, producing more mutations and therefore giving more facultative and resistance ability to the virus.
3D Structures of Reverse transcriptase
Reverse transcriptase 3D structures