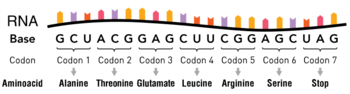We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Transfer RNA (tRNA)
From Proteopedia
(Redirected from TRNA)
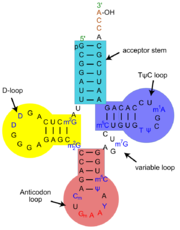
tRNA or transfer RNA plays a key role in translation, the process of synthesizing proteins from amino acids in a sequence specified by information contained in messenger RNA[1][2]. During this process, triplets of nucleotides (codons) of the messenger RNA are translated according to the genetic code into one of the 20 amino acids. tRNAs serve as the dictionary in this translation process. They contain a specific triplet nucleotide sequence, the anticodon, and they get attached to a specific (cognate) amino acid. During protein synthesis by ribosomes, tRNAs deliver the correct amino acids through interactions of their anticodon region with the complementary codons on the messenger RNA. Apart from their distinct anticodon regions, different tRNAs have very similar structures, allowing them to all fit into the tRNA-binding sites on the ribosome.
| |||||||||||
3D Structures of tRNA
Updated on 23-September-2022
See Also
References
- ↑ Biochemistry 5th ed. Berg JM, Tymoczko JL, Stryer L. New York: W H Freeman; 2002. Section 29.1 "Protein Synthesis Requires the Translation of Nucleotide Sequences Into Amino Acid Sequences" retrieved on 10/31/2018 from [1]
- ↑ Molecular Biology of the Cell. 4th ed. Section "From RNA to Protein" retrieved on 10/31/2018 from [2]
- ↑ Kim SH, Suddath FL, Quigley GJ, McPherson A, Sussman JL, Wang AH, Seeman NC, Rich A. Three-dimensional tertiary structure of yeast phenylalanine transfer RNA. Science. 1974 Aug 2;185(4149):435-40. PMID:4601792
- ↑ Kim SH, Sussman JL, Suddath FL, Quigley GJ, McPherson A, Wang AH, Seeman NC, RICH A. The general structure of transfer RNA molecules. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4970-4. PMID:4612535
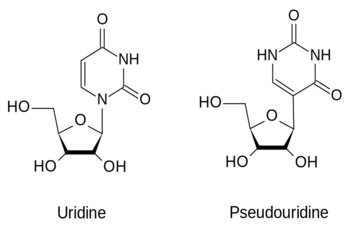
- ↑ Motorin Y, Helm M. tRNA stabilization by modified nucleotides. Biochemistry. 2010 Jun 22;49(24):4934-44. PMID:20459084 doi:10.1021/bi100408z
Reference for the structure
- Shi H, Moore PB. The crystal structure of yeast phenylalanine tRNA at 1.93 A resolution: a classic structure revisited. RNA. 2000 Aug;6(8):1091-105. PMID:10943889
Proteopedia Page Contributors and Editors (what is this?)
Karsten Theis, Wayne Decatur, Michal Harel, Frédéric Dardel, Ann Taylor, Joel L. Sussman, Alexander Berchansky
Categories: Trna | Topic Page | Translation | Modification | RNA | Amino acid